Clinical study on
chronic spontaneous urticaria
Do you suffer from small red wheals on your skin that are very itchy? These symptoms are not due to a trigger?Chronic spontaneous urticaria (hives) usually occurs over a period of more than 6 weeks.
We are looking for patients who suffer from chronic spontaneous urticaria.
Contact us.
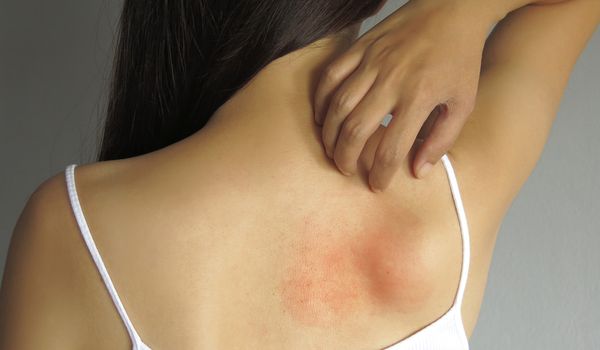
Possible symptoms and consequences of the disease:
- Hives on the skin
- Itching and burning
- Angioedema/ severe swelling
- Sleep disorders
- Listlessness
- Depression and anxiety
What we offer
We are looking for participants in our clinical trial who have these symptoms. We will help you relieve symptoms and gain a better understanding of your condition and possible treatment options.
You will receive comprehensive free study-related treatment from our specialists.
Treatment and more
Important info
Treatment
Therapy is with a monoclonal antibody or placebo via subcutaneous injection. Patients:inside are randomly divided equally into 2 groups. Since this is a placebo-controlled study, it is possible that you may receive an injection without novel active ingredients instead of the drug in the first period. In any case, however, you will receive the drug hereafter and have intensive treatment by experienced physicians throughout the study period.
Time required
Maximum approx. 9 months: The study drug is administered every 2 weeks and a total of 6 times. Participants are examined and monitored at regular intervals over the 12-week treatment period. Subsequently, some patients have the option of moving to an open-label phase. During this phase, you will receive the investigational drug with active ingredient every two weeks for a total of 12 weeks. This is followed by a 10-week follow-up period during which the patients continue to receive medical care.
Therapy is with a monoclonal antibody or placebo via subcutaneous injection. Patients:inside are randomly divided equally into 2 groups. Since this is a placebo-controlled study, it is possible that you may receive an injection without novel active ingredients instead of the drug in the first period. In any case, however, you will receive the drug hereafter and have intensive treatment by experienced physicians throughout the study period.
Maximum approx. 9 months: The study drug is administered every 2 weeks and a total of 6 times. Participants are examined and monitored at regular intervals over the 12-week treatment period. Subsequently, some patients have the option of moving to an open-label phase. During this phase, you will receive the investigational drug with active ingredient every two weeks for a total of 12 weeks. This is followed by a 10-week follow-up period during which the patients continue to receive medical care.
Clinical study: Chronic spontaneous urticaria
Participation criteria for study admission
Check here if you might be eligible for the chronic spontaneous urticaria study:
- Minimum age 18 years
- Diagnosis of chronic spontaneous urticaria with moderate to severe course
- No pregnancy during the study
- Exclusion in case of therapy with another biologic or chemotherapy during the study
Do these criteria apply to you? If so, you may be eligible for our clinical trial.
Please contact:
Charité University Medicine Berlin
Head of study: Prof. Dr. Martin Metz
E-mail: hautsache-ifa@charite.de
Phone: 030 450 518 046
FAQ Clinical Studies
Frequently asked questions
A clinical trial is "any investigation involving human subjects", that tests new medical treatmentsdrugs or therapies to determine their safety and efficacy.
Clinical trials are crucial for the development and validation of new medical approaches, to enable advances in healthcare and improve existing treatments.
Clinical trials are conducted not only in hospitals, but also by physicians in private practice. Pharmaceutical companies are often the clients of such studies, but universities or hospitals can also initiate such studies. An educational interview will be conducted prior to potential participation. Here you will be informed about goals, methods, advantages and possible risks. After sufficient time to think about it, you can decide whether you want to participate in the clinical trial. Participation is always voluntary and can be terminated at any time without giving reasons. The initial consultation will be followed by the screening visit, during which your eligibility for the study will be assessed. If the assessment is positive, the baseline visit occurs, in which baseline values are recorded and then the study medication or a placebo is administered. During the treatment phase, regular visits are scheduled to monitor your health, the effectiveness of the medication, and any side effects. After the treatment phase, there is a follow-up phase to evaluate the long-term effects and safety of the medication.
Yes, the safety of the participants is a top priority. Clinical trials are carefully planned and monitored to minimize potential risks. Prior to participation, researchers must disclose all risks and potential benefits of a study, and participation is always on a voluntary basis.
