All clinical studies
Find a study. We have compiled a list of our currently running clinical studies for you, so that you can quickly and easily find out about studies that suit you.
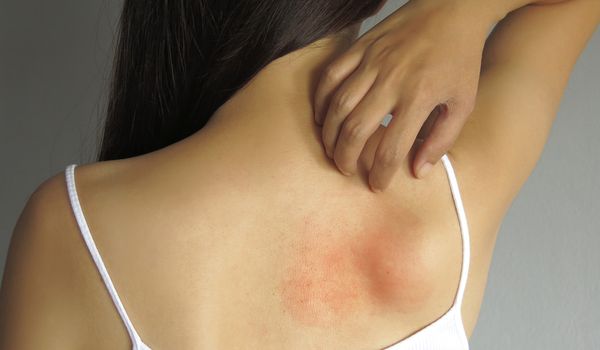
Skin diseases and allergies
What dermatological clinical pictures are there and how do symptoms manifest themselves? Find out here about possible allergies and skin diseases.
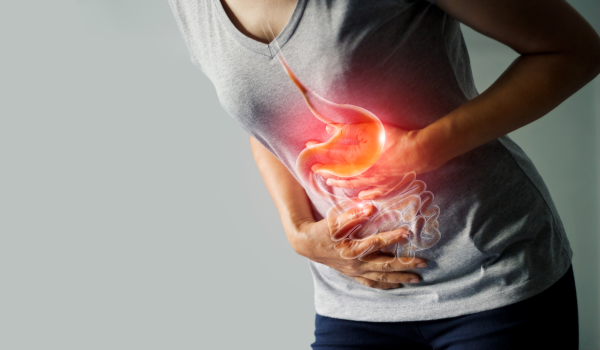
Gastrointestinal diseases
Find out about possible clinical pictures of gastrointestinal diseases and their most common causes and symptoms.
Metabolism and hormone disorders
The best-known metabolic and hormonal diseases include diseases of the thyroid gland and diabetes mellitus, also known as diabetes.